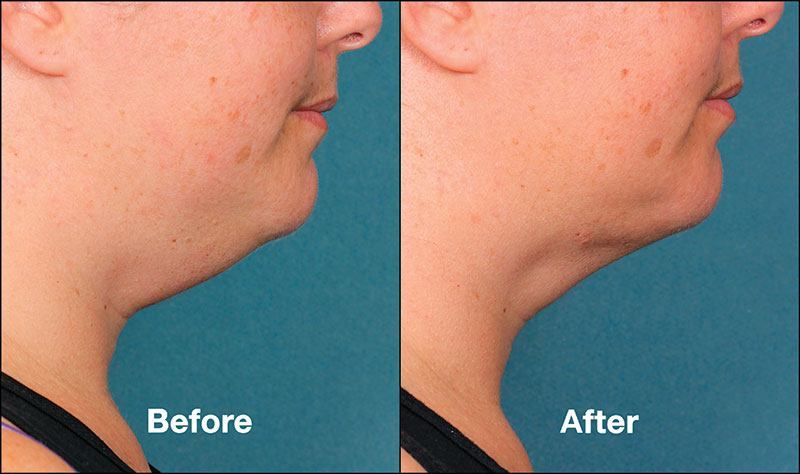
Kybella
Conveniently located to serve Hopkinsville and Christian County
No surgery – no more double chin.
Does your double chin have you down? Kybella® may be your answer! Kybella®, the only FDA approved treatment for submental fullness, is a non-surgical procedure injected into the “double chin” to breakdown stubborn fat.
 Earn future Savings on your purchase of Kybella® today with Allé by Brilliant Distinctions®
Earn future Savings on your purchase of Kybella® today with Allé by Brilliant Distinctions®
What is KYBELLA™ (deoxycholic acid) injection?
• KYBELLA™ can contour your profile & reduce the fat under your chin.
• KYBELLA™ can improve the appearance of moderate to severe convexity or fullness associated with submental fat (double chin) in adults.
• The safe and effective use of KYBELLA™ has not been established outside the submental area and is not recommended.
What is submental fullness?
• Submental fullness, sometimes referred to as “double chin,” is a common, yet undertreated facial aesthetic condition. It can detract from an otherwise balanced and harmonious facial appearance – leading to an older and heavier look.
• According to a 2015 survey by the American Society for Dermatologic Surgery (ASDS), 67% of consumers are bothered by submental fullness—nearly as many as those bothered by lines and wrinkles around the eyes (69%).
• It is a common, yet undertreated facial aesthetic condition that can:
- Detract from an otherwise balanced and harmonious facial appearance
- Lead to an older and heavier look
- Impact a broad range of adults, including both women and men
- Be caused by aging, genetics and/or weight gain
How does KYBELLA™ work?
• When injected into subcutaneous fat under the chin, KYBELLA™ can cause the destruction of fat cells. Once destroyed, those cells cannot store or accumulate fat.
What are the results of KYBELLA™?
• Many patients also reported improvement in the visual and emotional impact of submental fat when asked how happy, bothered, self-conscious, embarrassed, old and overweight they felt following treatment in relation to the amount of their submental fat.
What are the side effects with KYBELLA™?
• KYBELLA™ has been the focus of a global clinical development program involving over 20 clinical studies with more than 2,600 patients worldwide, of which over 1,600 have been treated with KYBELLA™.
• The most common adverse reactions were edema/swelling, hematoma/bruising, pain, numbness, erythema and induration.
• KYBELLA™ is manufactured through a highly-controlled, FDA-regulated process and approved facility to ensure patient safety.